As the pandemic began traversing the globe, Italy was one of the hardest hit countries. The devastation it caused as the medical community grappled with early treatment methods was unimaginable. Italy instilled one of the first nationwide lockdowns as it attempted to slow the staggering numbers of hospitalizations and deaths, causing unprecedented disruptions in its nation’s business and economic landscapes.
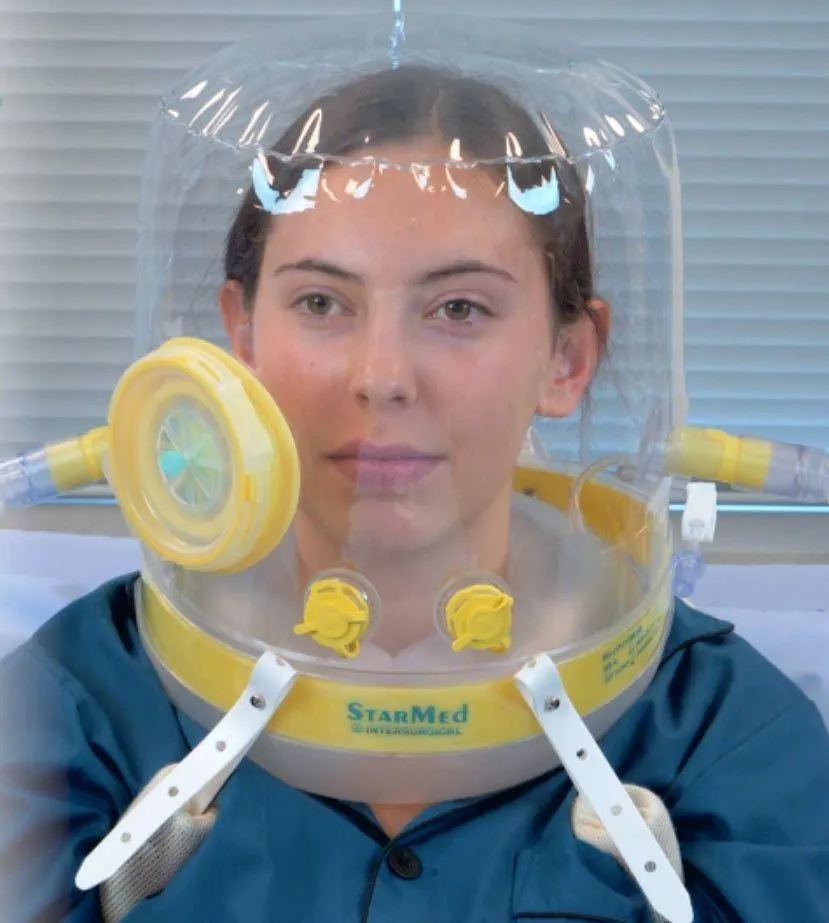
Among the many who were impacted was Intersurgical Respiratory Systems, which produces a wide range of high-quality medical devices for respiratory support, including Patient Interface Hoods. These products use non-invasive mechanical ventilation (NIV) — that is, assisted breathing, administered through a face mask, nasal mask or helmet.

Ironically, these devices which are used to treat acute hypoxemic respiratory failure had been identified recently as an effective alternative for some COVID-19 patients to ventilators, whose demand had spiked to the point of scarcity. While these hoods aren’t an option for every patient, depending on the severity of their symptoms, they have the potential to help those in respiratory distress, ensuring that more invasive ventilators remain available for the critically ill. Virtually overnight, Intersurgical’s demand for its life-saving product increased significantly.
Intersurgical quickly realized it could not sustain the required demand levels, as everything from materials and suppliers to distribution and labour was being impacted by the embargo and state of emergency imposed across Italy. Members of Harmac’s Buffalo and Ireland teams met with Intersurgical’s (virtually) in early April to determine the full scope of their needs and product specifications. Then, we got to work.
We adapted a clean room at our Castlerea, Ireland facility and ramped up high-volume production quickly to support this critical product’s local and global demand. We accelerated the manufacturing and sourcing of the components required in order to manufacture these intricate hoods. We purchased and adapted the equipment required to make the product, installed and validated the production line and sourced materials from various locations. We provided samples to the customer for evaluation and shipped our first kits to Italy in early July.
And we did it all in 61 days.
“Normally, it would take 6 to 12 months at minimum to launch a product like this,” explained Managing Director Mick McEnroe, who leads our Harmac Ireland team. “It’s not every company that can do that. Harmac has class-leading capabilities in both its Buffalo and Ireland facilities, which collaborated from an engineering, tooling and manufacturing design standpoint to ensure this project was ramped as aggressively as possible. It’s a major differentiator between us and competitors, and it’s inspiring to help a customer under these circumstances.”
The demand for Intersurgical’s StarMed hoods is expected to increase substantially over the next 12 months. In fact, Intersurgical received FDA Emergency Usage Authorization for their hoods on August 14, 2020.
We’re honored to have been chosen as their partner in this important project.
“We had numerous proposals from potential partners, but Harmac’s was by far the best,” explained Intersurgical Managing Director Charles Bellm, “and they got to manufacturing the final product very quickly.”
All of us at Harmac are extremely proud of what we were able to accomplish through this project because it epitomizes our mission: to change the lives of patients, employees and the communities in which we work. We assisted a new customer during its hour of need. We leveraged our medtech expertise and partnerships to benefit our employees and communities. Most importantly, we’re producing a product that will literally save lives of countless patients, including those who are struggling with this terrible virus.
How can Harmac help your business? Contact us to find out.